Research Progress
Qin Wenxin team released a long review elucidating the application and development trend of high-throughput functional genetic screening using CRISPR/Cas9 in liver cancer research
Date:2021-06-24On June 23, a long review of Tan Wenxin team from Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine and Shanghai Cancer Institute (SCI), titled “Exploring liver cancer biology through functional genetic screens” was published in the Nature Reviews Gastroenterology & Hepatology [1]. The review comprehensively summarized the research advances in the CRISPR associated protein 9 (Cas9) functional genetic screening technique in liver cancer, illustrated the current progress in exploring the development and progression of liver cancer and its resistance mechanism using this technique and new strategies to develop novel treatment targets, and proposed insights and reflections for the bottlenecks and future development trend of liver cancer research.

Liver cancer is a multi-factor-mediated malignancy that involves multiple genes and has complicated pathogenesis. There are more than 840 thousand new cases and more than 780 thousand deaths of primary liver cancer annually worldwide [2], about half of which occur in China, making liver cancer a highly prevalent cancer in China [3]. Hepatocellular carcinoma, a major type of primary liver cancer, accounts for about 85-90% of cases of liver cancer [4]. Surgical resection is an effective treatment for early liver cancer. Due to the difficulty in early diagnosis of liver cancer and its rapid progression, most patients have reached the middle and advanced stages when diagnosed, losing the opportunity for surgery, and resulting in a 5-year survival rate of only about 15-18%.
In recent years, the mutations of key genes driving the development and progression, including TERT promoter, TP53, CTNNB1, ARID1A, and AXIN1 have been identified as the high-throughput sequencing technique has been developed quickly and comprehensively applied [5]. However, most mutations, different from BARF mutation in melanoma or EGFR mutation in lung cancer, fail to directly serve as an effective drug target in liver cancer. Multi-kinase target inhibitors sorafenib and lenvatinib, the first-line drugs for the current clinical treatment of liver cancer, can only provide limited survival benefit [6]. Immune checkpoint blockade therapy has attracted extensive attention in the field of oncotherapy over recent years [7]. The latest IMbrave150 trial results have shown that the combination of atezolizumab [a programmed death-ligand 1 (PD-L1) inhibitor] and bevacizumab (an angiogenesis inhibitor) significantly improves the prognosis of patients with advanced liver cancer, but the response rate is only 27.3% [8]. Recently, a randomized, open-label, phase 2-3 multi-center clinical trial conducted by Fan Jia team from Zhongshan Hospital Affiliated to Fudan University suggested that sintilimab (a PD-1 inhibitor) plus IBI305 (a bevacizumab biosimilar) has more significant overall survival and progression-free survival benefits than sorafenib, with acceptable safety, providing a treatment option for patients with unresectable liver cancer [9].
Despite some breakthroughs in the exploration of treatment for liver cancer over the past decade, there is still a critical issue most urgently to be solved in the field of liver cancer research that the effective treatment means are lacking for advanced liver cancer, and the efficacy of the current standard therapy remains to be further raised [10]. In this review, the author systematically described the principles, advantages and disadvantages of different high-throughput functional genetic screening platforms and elucidated plenty of breakthroughs and progress achieved by means of high-throughput functional genetic screening platforms from the three aspects: identification of the driving genes for the development and progression of liver cancer, exploration of drug sensitivity and resistance mechanism of liver cancer as well as development of new targets for the treatment of liver cancer. Moreover, the possible challenges and potential opportunities were further analyzed in terms of translating advancements into clinical treatment schemes. Finally, the author pointed out that CRISPR-Cas9 high-throughput functional genetic screening platforms hold large application value and potential for the research of tumor heterogeneity, metastasis and recurrence, and immunity, and these studies will ultimately help deepen our understanding of liver cancer and develop novel potential treatment strategies.
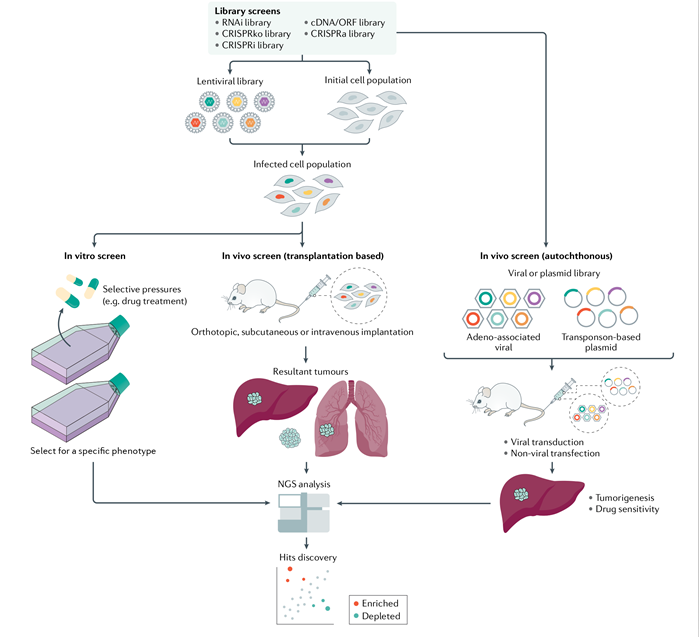
CRISPR-Cas9 functional genetic screening technique in liver cancer,
[1] Wang C, et al. Exploring Liver Cancer Biology through Functional Genetic Screens. Nat Rev Gastroenterol Hepatol. 2021 June 23, Online ahead of print.
[2] Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68:394-424.
[3] Allemani C, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018, 391(10125):1023-1075.
[4] Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019, 380:1450-1462.
[5] Zucman-Rossi J, et al. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015, 149(5):1226-1239.
[6] Kudo M, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018, 391(10126):1163-1173.
[7] Finn RS, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38:193-202.
[8] Finn RS, et al. IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020, 382(20):1894-1905.
[9] Ren Z, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021 Jun 15, Online ahead of print.
[10] Llovet JM, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016, 2:16018.
[11] Shalem O, et al. High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet. 2015,16:299-311.
[12] Wang C, et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019, 574(7777):268-272.
[13] Wang C, et al. CDK12 inhibition mediates DNA damage and is synergistic with sorafenib treatment in hepatocellular carcinoma. Gut. 2020, 69(4):727-736.
[14] Wang C, et al. A CRISPR screen identifies CDK7 as a therapeutic target in hepatocellular carcinoma. Cell Res. 2018, 28(6):690-692.
[15] Wang C, et al. Phospho-ERK is a biomarker of response to a synthetic lethal drug combination of sorafenib and MEK inhibition in liver cancer. J Hepatol. 2018, 69(5):1057-1065.
link:https://www.nature.com/articles/s41575-021-00465-x
